-
 Infection, Immunity & InflammationIn the early 90ies of the last century experiments on the cellular response mechanisms of interferons led to the identification of the JAK-STAT pathway as a paradigm for signaling from the cell surface to chromatin. JAK-STAT controls the development and activity of hematopoietic cells. They provide inflammatory and immune stimuli and thereby regulate host-pathogen interactions, tumor surveillance and inflammatory processes.MORE
Infection, Immunity & InflammationIn the early 90ies of the last century experiments on the cellular response mechanisms of interferons led to the identification of the JAK-STAT pathway as a paradigm for signaling from the cell surface to chromatin. JAK-STAT controls the development and activity of hematopoietic cells. They provide inflammatory and immune stimuli and thereby regulate host-pathogen interactions, tumor surveillance and inflammatory processes.MORE -
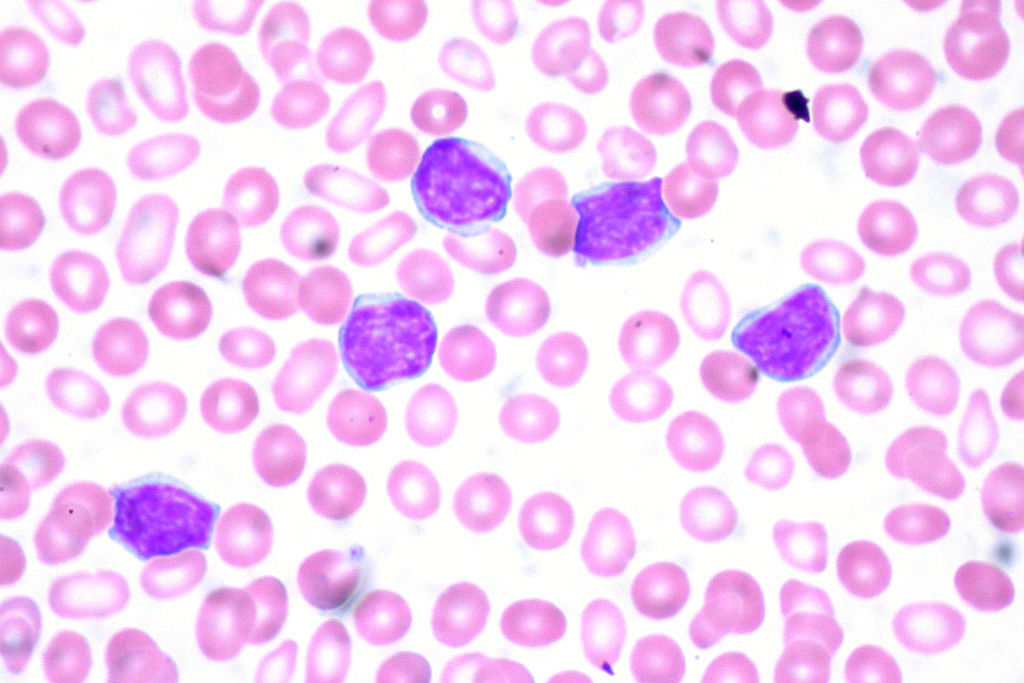

 Hematopoietic MalignanciesConstitutive JAK and/or STAT activation is found in most myeloproliferative neoplasms and in many other hematopoietic malignancies. It is frequently a biomarker of poor prognosis. The underlying molecular aberrations include activating mutations in or overexpression of signaling components (cytokine receptor, JAK, STAT), or rare JAK fusion proteins.MORE
Hematopoietic MalignanciesConstitutive JAK and/or STAT activation is found in most myeloproliferative neoplasms and in many other hematopoietic malignancies. It is frequently a biomarker of poor prognosis. The underlying molecular aberrations include activating mutations in or overexpression of signaling components (cytokine receptor, JAK, STAT), or rare JAK fusion proteins.MORE -
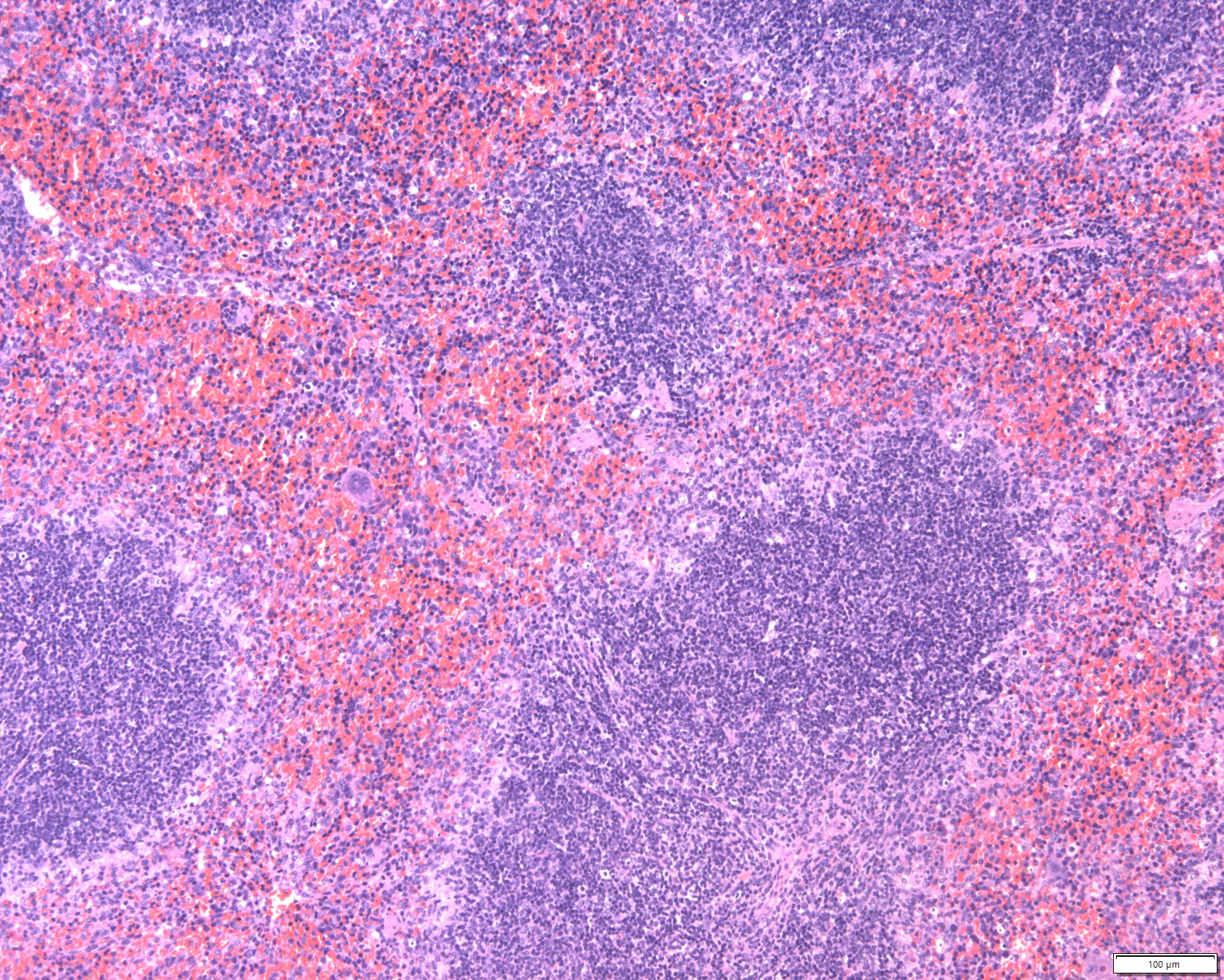

 Homeostatic Cell Type-Specific RegulationCellular and tissue homeostasis is the result of variables being regulated on chromatin level such that the internal environment remains stable and fairly constant even though the external environment varies. Cell type-specific homeostatic chromatin landscapes are maintained by tonic (constitutive) signals provided by auto- and paracrine acting cytokines and growth factors as well as by cell-cell contacts. All SFB members contribute to the generation of a map of the JAK-STAT-shaped chromatin landscape of various hematopoietic and structural cell types under homeostatic in comparison to stressed or diseased conditions.MORE
Homeostatic Cell Type-Specific RegulationCellular and tissue homeostasis is the result of variables being regulated on chromatin level such that the internal environment remains stable and fairly constant even though the external environment varies. Cell type-specific homeostatic chromatin landscapes are maintained by tonic (constitutive) signals provided by auto- and paracrine acting cytokines and growth factors as well as by cell-cell contacts. All SFB members contribute to the generation of a map of the JAK-STAT-shaped chromatin landscape of various hematopoietic and structural cell types under homeostatic in comparison to stressed or diseased conditions.MORE -

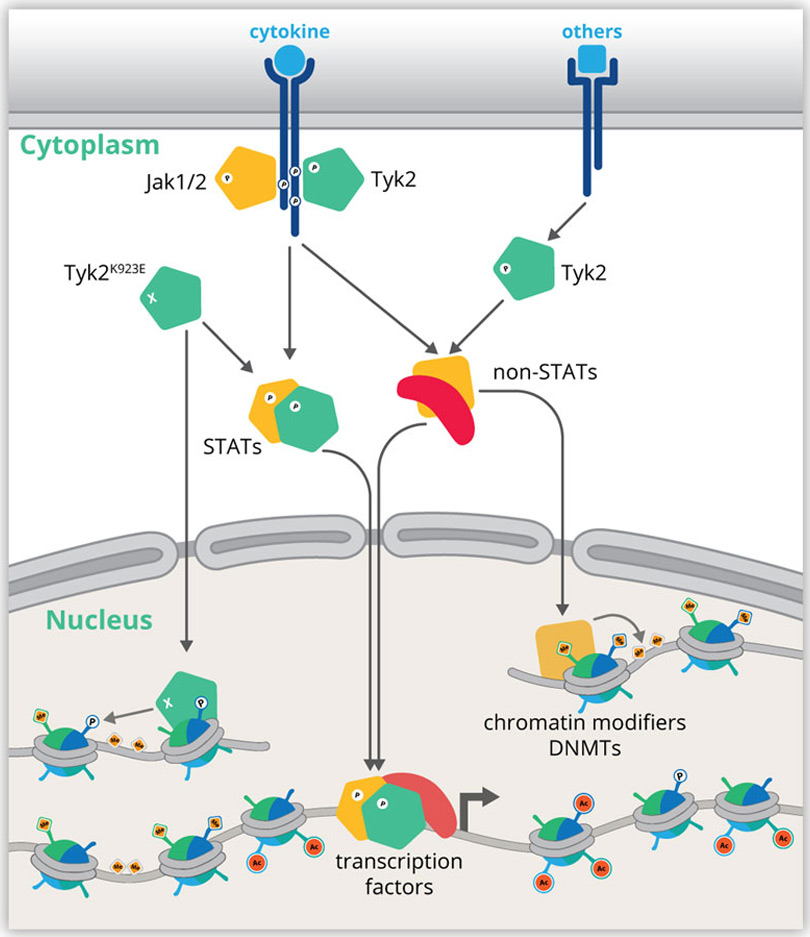
 The Epigenome of JAK-STATRecently molecular genetics research focused on global aspects of gene control and the underlying hierarchies. Our proposed projects aim to place the interactomes of JAKs and STATs into the emerging global chromatin landscape. Genome-wide analyses integrate the various layers of RNA, DNA and protein dynamics to pinpoint key regulators of pathways and pathway hierarchies that drive or prevent disease. We aim to determine the impact of JAKs and STATs on 3D genome architecture, landscapes of chromatin modifications, DNA methylation and transcription factor binding.
The Epigenome of JAK-STATRecently molecular genetics research focused on global aspects of gene control and the underlying hierarchies. Our proposed projects aim to place the interactomes of JAKs and STATs into the emerging global chromatin landscape. Genome-wide analyses integrate the various layers of RNA, DNA and protein dynamics to pinpoint key regulators of pathways and pathway hierarchies that drive or prevent disease. We aim to determine the impact of JAKs and STATs on 3D genome architecture, landscapes of chromatin modifications, DNA methylation and transcription factor binding.
The Viennese JakStat Consortium is worldwide one of the leading research groups studying signal transmission by the Janus kinases (JAKs) and signal transducers and activators of transcription (STATs). For more than a decade the Consortium has been funded as a Special Research Program (SFB) by the Austrian Science Fund FWF and has made substantial contributions to the current understanding of the functions of JAKs and STATs in homeostasis and disease.
This progress has been fuelled largely by the generation and analysis of advanced transgenic mouse models for a number of human disorders, such as haematopoietic malignancies as well as infectious, inflammatory and metabolic diseases. Today the JAK-STAT pathway is recognised as one of the twelve core pathways in the initiation and progression of cancer and a central communication node for the immune system.
LATEST NEWS
6th International Conference on Cytokines in Cancer
Sylvia Knapp receives Prize of the City of Vienna
Ulrich Kalinke
Seven internationally competitive research teams in Vienna join forces to deploy next-generation sequencing and proteome technologies based on strong bioinformatics competence. We share the vision of advancing the knowledge of chromatin architectures that govern (patho)-physiological processes. JAK-STAT serves as a paradigm to identify hierarchies, key players and co-factors shaping the chromatin landscapes of hematopoietic immune cells and structural non-immune cells under healthy and diseased conditions. The chromatin architecture of these cells defines their transcriptional profiles that are either a prerequisite for, or a consequence of disease.
Our mission is to discover how the JAK-STAT pathway drives the complex reorganization of the genome when cells undergo specialization or pathophysiological changes. We pursue our hypothesis that JAK-STAT signals contribute to extensive transcriptome changes underlying cell growth and immunity on the one hand, and runaway activation or mutation causing cancer and derailed immune responses on the other. Fundamental understanding of these processes requires definition of the ‘monarchs’ at the top of the hierarchy of chromatin remodelling and their relationship to JAK-STAT. Understanding the dynamics and reconfiguration of chromatin will allow us to compare human diseases with models in genetically engineered mice, in which we can test new therapeutic concepts.